ELI LILLY & CO (NYSE:LLY) reported third-quarter 2025 financial results that significantly exceeded analyst expectations, triggering a notable pre-market rally. The pharmaceutical giant demonstrated exceptional commercial execution, particularly within its cardiometabolic portfolio, leading to a substantial upward revision of its full-year outlook.
Earnings and Revenue Performance
The company’s financial performance for the quarter was marked by a substantial beat on both the top and bottom lines. The results underscore the immense demand for its key products.
- Revenue: Reported Q3 revenue reached $17.60 billion, surpassing analyst estimates of approximately $16.20 billion.
- Earnings Per Share: Non-GAAP EPS came in at $7.02, handily beating the consensus estimate of $5.92.
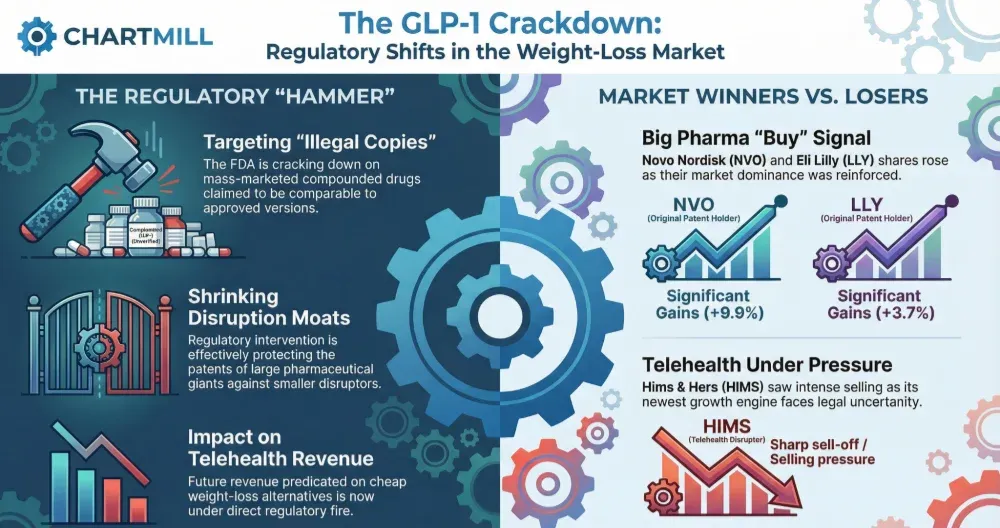
This strong outperformance was primarily fueled by what the company refers to as its "incretin portfolio," specifically the diabetes treatment Mounjaro and the obesity drug Zepbound. The 54% year-over-year revenue growth highlights the blockbuster status of these therapies and their dominant position in the GLP-1 market.
Updated Financial Guidance
Bolstered by the quarter's strength, management raised its full-year 2025 revenue guidance. The company now anticipates revenue of approximately $63.25 billion at the midpoint. This updated forecast sits about 2.6% above the prior analyst consensus estimate of $61.63 billion, signaling continued confidence in the company's growth trajectory for the remainder of the year. The raised guidance serves as a positive signal to the market about sustained demand and operational momentum.
Market Reaction
The market responded positively to the earnings beat and raised outlook. In pre-market trading following the announcement, Eli Lilly & Co's stock advanced approximately 3.4%. This immediate price action reflects investor approval of the company's ability to not only meet but significantly exceed financial targets. The strong performance in the face of high expectations suggests underlying operational strength and robust product demand that the market views favorably.
Pipeline and Operational Highlights
Beyond the financial figures, the earnings release highlighted significant progress in research and development. CEO David A. Ricks noted that the company advanced orforglipron, an oral obesity treatment, through four additional Phase 3 trials, with plans for global regulatory submissions by the end of the year. The company also secured U.S. FDA approval for a new product, Inluriyo (imlunestrant). Furthermore, Eli Lilly is actively expanding its manufacturing footprint with new facilities in Virginia and Texas and an expansion in Puerto Rico, addressing one of the key constraints on its growth: production capacity for its high-demand drugs.
For a detailed breakdown of historical earnings and future analyst estimates, you can review the data here.
Disclaimer: This article presents a summary of recently reported financial information and is for informational purposes only. It does not constitute investment advice or a recommendation to buy or sell any security.