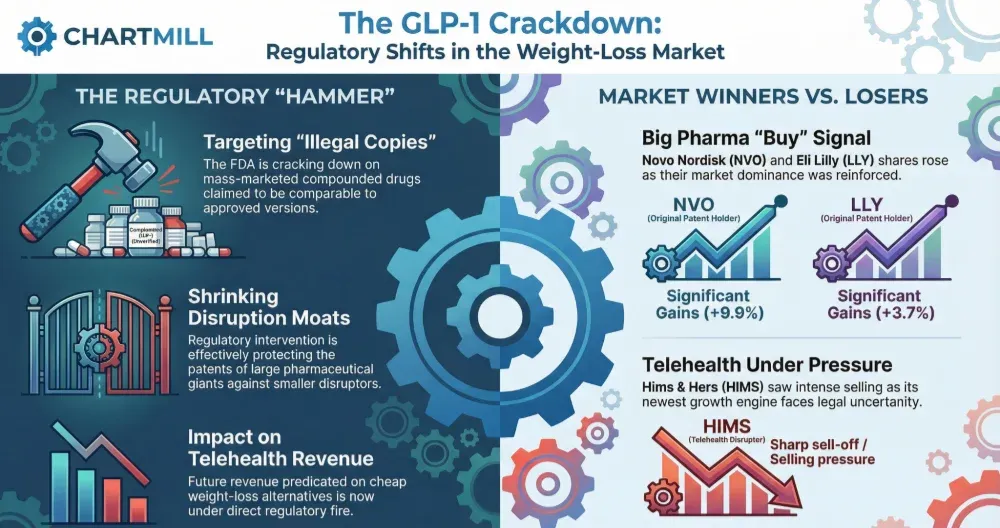
In a market currently obsessed with the multi-billion dollar GLP-1 "weight-loss gold rush," we just saw how quickly the regulatory hammer can fall.
While the Dow was busy popping champagne over its 50,000 milestone, investors in the telehealth space were hit with a cold reality check that proves disruption isn't always a smooth ride, especially when the federal government gets involved.
The FDA’s "Swift Action" on "Illegal Copies"
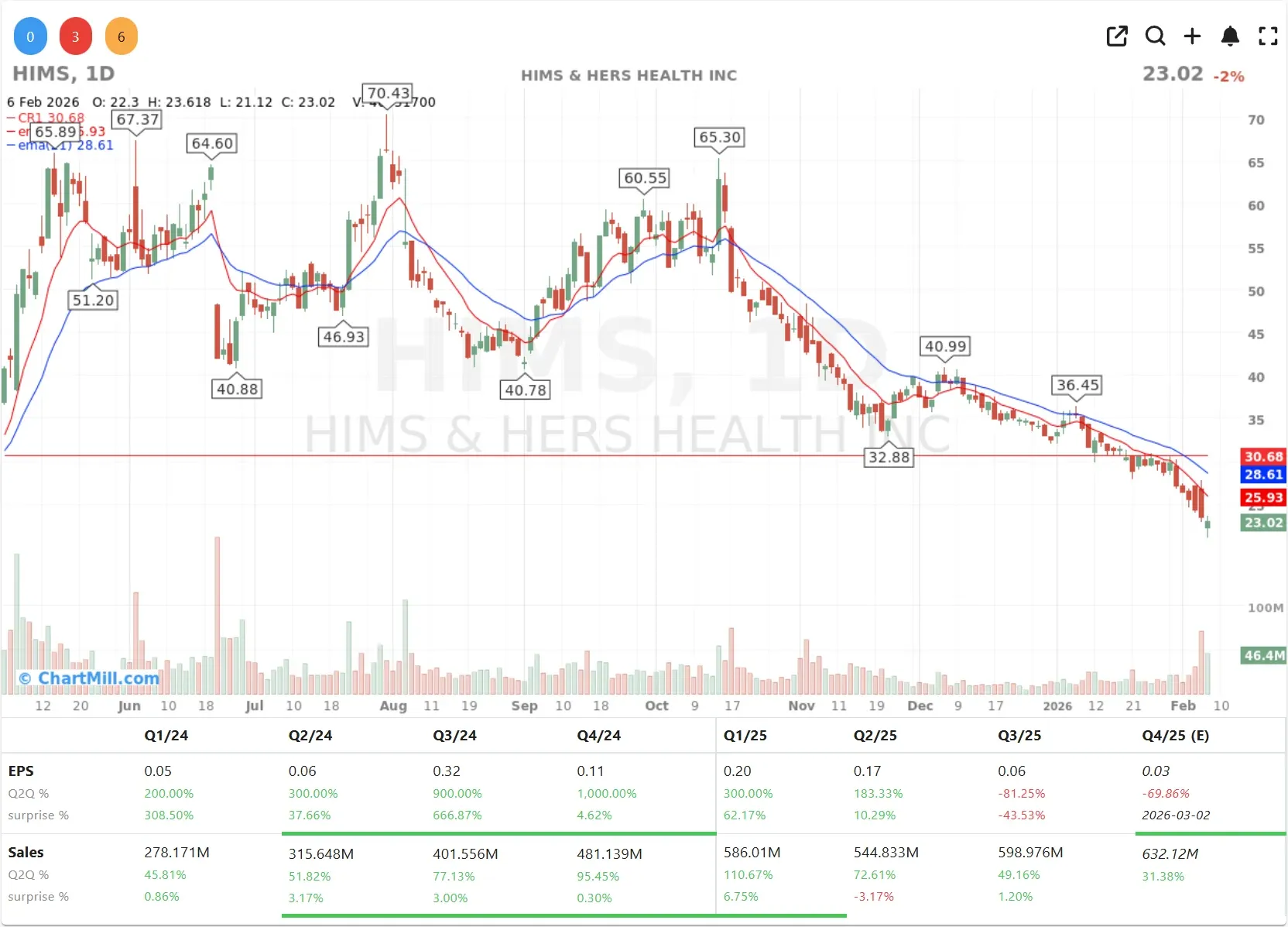
The primary catalyst for the sharp decline in Hims & Hers (HIMS | -14.12 premarket%) is a direct and rather aggressive stance taken by the U.S. Food & Drug Administration (FDA).
Late Thursday, FDA Commissioner Marty Makary took to social media to announce that the agency will take "swift action" against companies that are marketing illegal copies of drugs on a large scale. Crucially, the FDA is targeting firms that claim these compounded versions are "comparable" to the officially approved products from the pharmaceutical giants.
Hims & Hers recently made headlines by launching a version of the popular weight-loss drug Wegovy. While compounding is often a legal gray area during drug shortages, the FDA's language suggests they are no longer willing to look the other way as these "copies" become mass-marketed commodities.
This has spooked the market, as investors realize that a significant portion of the company’s future revenue growth - predicated on these cheaper weight-loss alternatives - is now under direct regulatory fire.
A Tale of Two Sides: Winners and Losers
While Hims & Hers closed 2% lower last Friday and is trading 14% lower in premarket today, the news was a massive "buy" signal for the original patent holders.
Novo Nordisk (NVO | +9.92%) and Eli Lilly (LLY | +3.66%) both saw significant gains as the FDA move effectively protects their multi-billion dollar moats.

This is a classic example of "regulatory capture" in action; the high barrier to entry provided by FDA approval is being reinforced, leaving the smaller "disruptors" out in the cold.
When a company is down double digits after hours while the Dow is celebrating 50,000 points, it tells you the market is fundamentally reassessing that specific company's ability to operate.
The Disruption Moat is Shrinking
This is a wake-up call for anyone betting on telehealth companies that play in the pharmaceutical margins.
The "Claude Effect" we saw earlier this week - where AI tools like Claude from Anthropic threatened the moats of data companies - is now being mirrored by the "FDA Effect" in healthcare. In both cases, investors are realizing that what they thought were defensible business models are actually quite fragile when faced with either technological or regulatory shifts.
Where are We Now?
Hims & Hers is currently being revalued not on its marketing prowess, but on its legal standing. The FDA’s pledge to act "swiftly" has effectively put a ceiling on the company's most lucrative new venture.
In my opinion, until we see how the FDA actually executes this action, Hims & Hers will remain a "catch a falling knife" scenario for most conservative portfolios.
The big pharma giants have won this round, and the milestone at Dow 50,000 won't do much to comfort those who bet on the compounded copycats.
Kristoff - ChartMill