First Disclosure of Global Interim Phase 2 Data for BioNTech and Bristol Myers Squibb PD-L1xVEGF-A Bispecific Antibody Pumitamig (BNT327/BMS986545) in Patients with Extensive-Stage Small Cell Lung Cancer Shows Encouraging Antitumor Activity
Provided By GlobeNewswire
Last update: Sep 8, 2025
MAINZ, Germany, and PRINCETON, USA, September 8, 2025 – BioNTech SE (Nasdaq: BNTX, “BioNTech”) and Bristol Myers Squibb Company (NYSE: BMY, “BMS”) today presented interim data from a global randomized Phase 2 trial (NCT06449209) evaluating pumitamig (also known as BNT327 or BMS986545), an investigational bispecific antibody targeting PD-L1 x VEGF-A, plus chemotherapy in patients with extensive-stage small cell lung cancer (“ES-SCLC”). The data, which are consistent with data presented at the European Lung Cancer Congress (“ELCC”) 2025 from a Phase 2 trial conducted in China (NCT05844150), showed encouraging anti-tumor responses with a positive trend in the secondary endpoint progression free survival. Pumitamig plus chemotherapy demonstrated a manageable safety profile with no new safety signals and a low discontinuation rate. The data are being presented today as a late-breaker oral presentation at the IASLC 2025 World Conference on Lung Cancer (“WCLC”) hosted by the International Association for the Study of Lung Cancer in Barcelona.
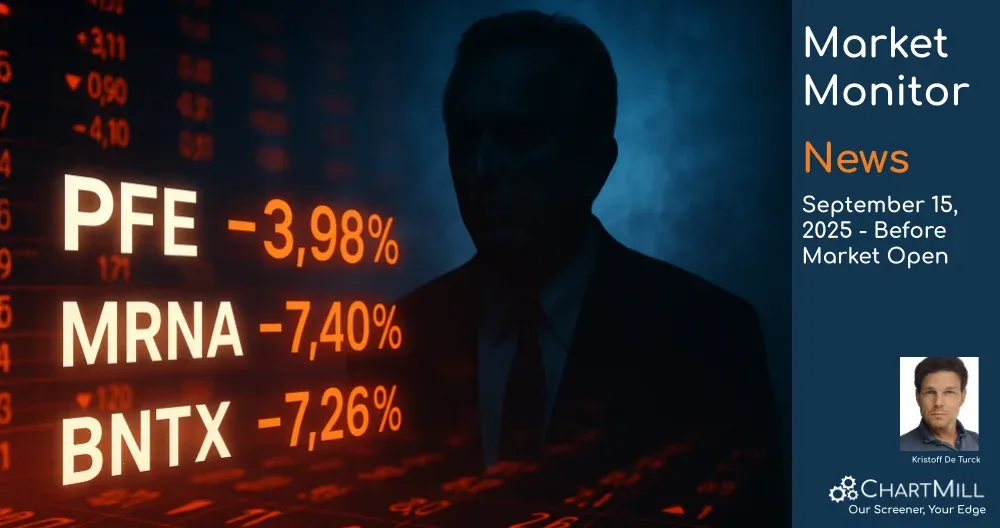
Read more at globenewswire.com99.75
+1.48 (+1.51%)
Find more stocks in the Stock Screener
BNTX Latest News and Analysis